Abstract
Background Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome that is typically fatal without treatment. Standardized treatment with etoposide and dexamethasone has improved survival, but approximately 15% of pediatric patients die in the first months after diagnosis and inadequate response requiring salvage therapy is frequent. Identifying at-risk patients promptly is likely to improve outcomes.
Methods We assessed early treatment-response laboratory markers to identify refractory HLH and risk of early mortality through a multi-institutional, retrospective study of children treated per HLH-1994 or HLH-2004 from 2008-2019. Baseline and biweekly data were obtained during the first 100 days of treatment and evaluated at specified timepoints using receiver-operating curves (ROC). To identify useful prognostic indicators, statistical significance was determined according to the first 31 days of treatment. Results were validated using odds ratios (OR), Kaplan-Meier survival analysis, and hazard ratios (HR). The endpoint was survival to bone marrow transplant (BMT) or approximately 1-year if no BMT was pursued.
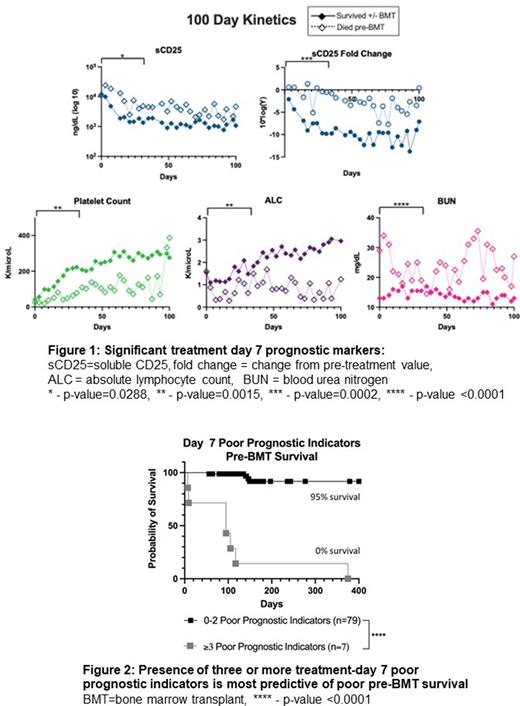
Results Eighty-nine patients met inclusion criteria. Pre-BMT mortality was 13.4% (n=12) and overall mortality was 25.8% (n=23). Markers obtained at treatment day 7 better-predicted outcome compared to baseline and most treatment day 14 markers. Unfavorable day 7 markers with significant discriminatory ability included fold-change from pre-treatment level in soluble CD25 (OR 31.25, 95%CI 4.03-197.7), absolute sCD25 (OR 23.25, 95%CI 2.53-306.6), platelet count (OR 27.6, 95%CI 5.44-114.3), absolute lymphocyte count (OR 14.5, 95%CI 3.43-52.86), and blood urea nitrogen (OR +infinity, 95%CI 5.44-infinity [all patients above the optimized threshold (20 ng/dL) died, denominator is zero]) (Figure 1). Interestingly, ferritin level or fold-change from baseline was a significant indicator only at day 14. The presence of three or more of the day 7 unfavorable markers was associated with 100% probability of pre-BMT mortality while 0-2 unfavorable markers was related with only 5% mortality up to BMT. (Figure 2).
Conclusions Five early prognostic laboratory indicators were identified in pediatric patients receiving etoposide-based therapy for HLH, these include not only some HLH-94 diagnostic criteria but also other laboratory parameters. Survival analysis identified that the presence of three or more unfavorable day 7 prognostic indicators is associated with significantly high risk of pre-BMT mortality and may indicate the need for early escalation of therapy.
Disclosures
Yacobovich:Novartis Israel: Consultancy. Zoref-Lorenz:Sobi: Consultancy. Jordan:Sobi, Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal